Environmental Gas Cell
New Developments In Small Molecule X-ray Crystallography
Nasty Things Happen To Nice Crystals
In March 2004 Station 9.8, the small molecule X-ray crystallography (SMX) facility, on the SRS underwent a substantial upgrade with the installation of the APEXII diffractometer (a three circle D8 goniometer with APEXII detector from Bruker-Nonius). This new hardware allowed data collection times to drop from 360 to 80 minutes for a full sphere of data (the whole unique diffraction pattern plus many equivalent reflections). This combined with software improvements and alternate scanning modes allow for even faster (though less commonly used) data collection methods to be employed, bringing the full sphere down to just over 14 minutes and a hemisphere (the unique diffraction pattern and less equivalents) down to approximately 11. With this increase in the data collection speed parametric and dynamic studies of systems became a reality.
Test Case

Sulfur dioxide (SO2) is a classic green house gas which is produced through the combustion of fossil fuels such as coal. Its uncontrolled release has potentially harmful effects on plant and wildlife through the formation of acid rain (via the reaction of SO2 with water in the atmosphere). The corrosive rain also has an impact on man made structures such as ancient monuments, statues and buildings. A more efficient use of the SO2 by its selective removal from flue gases and exhausts would be desirable.
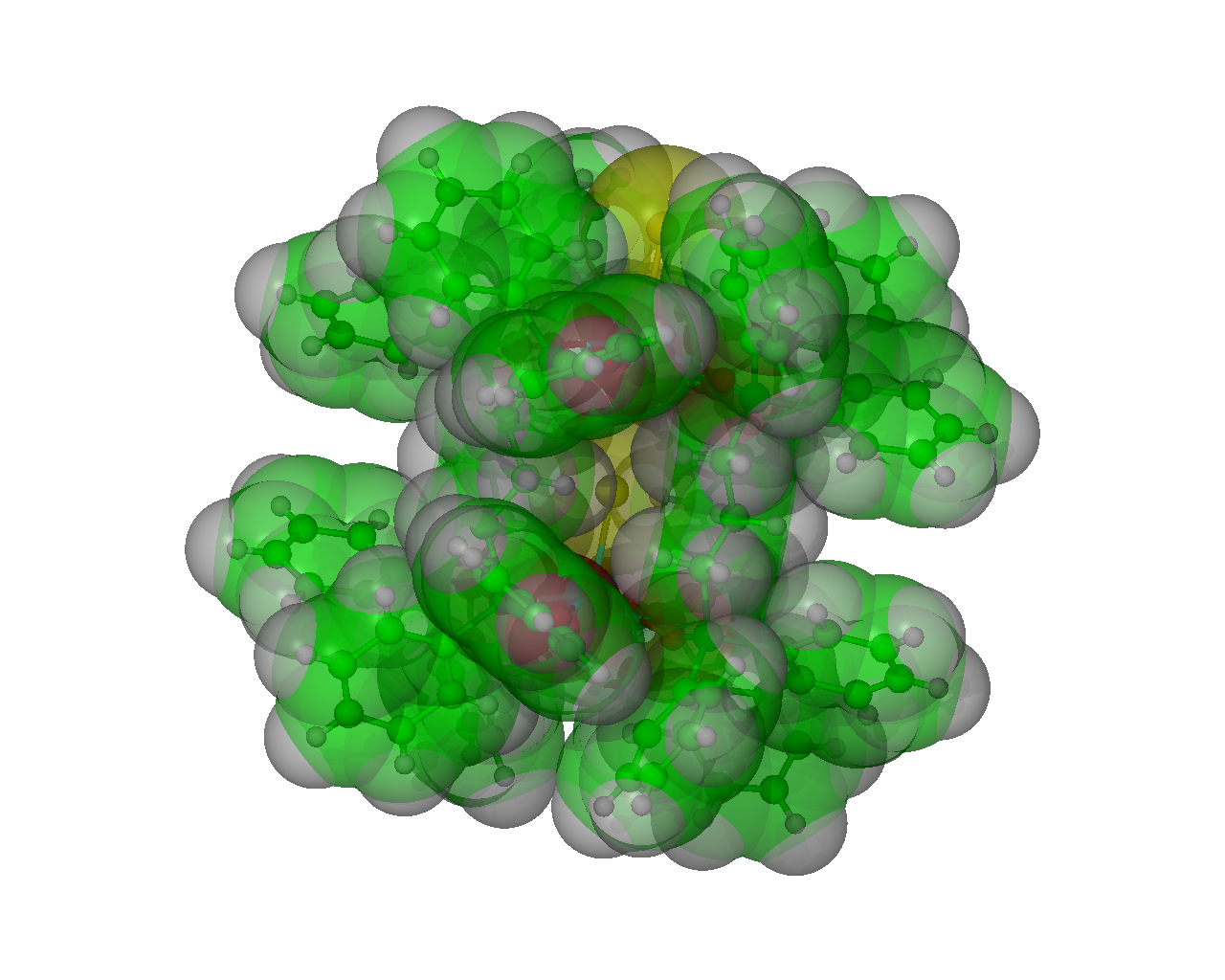
Work by McAuliffe and Pritchard lead to the discovery of a range of ‘Chinese Lantern’ complexes [XMn(µ-dppO2)4MnX]2+2X- [X = Cl, Br, I, NSC; dppO2 = 1,3-bis(diphenylphosphinoyl)propane] which showed the interesting property of reversible SO2 capture. Understanding how the SO2 is reversibly held in the lantern structure could lead to a system which would actively react and produce new commercial products via reversible uptake and release in a catalytic manner turning a harmful waste product into a new commercial product. These systems were reported to reversibly absorb 6 equivalents per lantern of SO2 when measured by thermogravametric analysis (TGA). However, due to the reversible and therefore “transient” nature of the SO2 adsorption it had not previously been possible to crystallographically characterise the preferential sites in the lattice for SO2 occupation.
All ‘Chinese Lanterns’ form as body centred tetragonal (a=b=14 c=26 Å), space group I4/m with the cation being centred on crystallographic C4hsites.

This system was therefore an ideal candidate for Warren and co-workers to undertake with the newly developed environmental cell. An experiment was designed to reproduce the TGA environment used to measure the bulk sample with the added step of in vacuo heating.

The work reported here is still on-going and the structures shown have been refined to moderate Rf (residual factor (Rf) is a mathematical comparison between the structural model and the actual data the lower the value the better!) values but further work is required on disorder (which is not shown). The structures constitute snapshots at key stages of the experiment.
A single crystal (approximately 0.02x0.04x0.04 mm) was selected which produced a diffraction pattern of sufficient quality for the experiment and provided enough information for full structure solution and refinement (structure A).

The sample was then heated for 2 hours on a ramping gradient from 10° to 130°C under vacuum insitu and 6 data collections collected to monitor the removal of solvent. Once solvent looked to be sufficiently removed the sample was cooled back to 10°C and a further data collection was undertaken. The data was solved and refined (structure B).

A SO2 and argon (Ar) mixed gas atmosphere was then slowly introduced into the cell by pressurising the gas cell input line with differing pressures of each gas. During this process a total of 18 data collections were undertaken to monitor the SO2 uptake. From the final data collection the displayed structure was solved and refined (structure C). The cell was then evacuated using a controlled exposure of vacuum, the data produced from this work is still being processed but initial unit cell parameters have been included for comparison.


Examination of a plot of the unit cell length of the a-axis in Å and the unit cell volume in Å3, clearly show changes across the range of conditions the crystal was exposed to, i.e., the physical parameters of the unit cell responded to the environment the crystal was exposed to. Furthermore these changes clearly map to logical changes within the unit cell. With the volume change of the cell perhaps most markedly showing the effect of removing solvent (Run 1 to 2) inserting guest molecules (Run 2 to 3) and the removal of guest molecules (Run 3 to 4).
By examination of the unit cell packing diagrams it can clearly be seen that on going from A-PACK to B-PACK the methanol moiety has been removed leaving a cavity which could host the incoming SO2 (this is corroborated by a reduction in the unit cell volume of approximately 110 Å3). The uptake of the SO2 moiety in C-PACK and the location of the cavities generated in the program PLATON (crystallography computer program) do not look to be coincidental and when combined with the apparent volume change (approximately 187 Å3) uptake of SO2 would appeared to be confirmed. Thus constituting the first single crystal X-ray diffraction structure of a ‘Chinese Lantern’ complex in which the location of the SO2 moiety has been determined.

The environmental gas cell is a departure from the traditional SMX experiment and is just part of the many new advancements to the experimental arsenal now available to the scientist. Whilst SMX is perhaps unique as a technique for giving 3-dimensional structural information when combined with the ability to change the crystals environment dynamically be that temperature, atmosphere, pressure or to expose a crystal to external stimulus, lasers, magnetic fields or electricity SMX becomes a truly unrivalled precision scientific tool. Two new designs for the cell are planned one an advancement on the current system to aid sample handling and the second design is to allow dynamic gas flow through the cell.
References
Cross WI, Godfrey SM, McAuliffe CA, Pritchard RG. Crystal engineering of microporous “Chinese-lantern” compounds to improve their ability to reversibly adsorb sulfur dioxide. Chem Commun. 2001, 0(18), 1764–5. http://dx.doi.org/10.1039/B101263K
PDF Version of this article from SRD Annual Report
PDF Version of the full SRD Annual Report
Atom Colour Key
- Green = Carbon
- White = Hydrogen
- Red = Oxygen
- Cyan = Manganese
- Yellow = Bromide
- Dark Green = Phosphorus
